From negative results to new discoveries
Article Highlights
- In a recent study in Plant Physiology, PRL researchers revealed a misconception that had been growing in plant science about one of the fundamental puzzle pieces of photosynthesis.
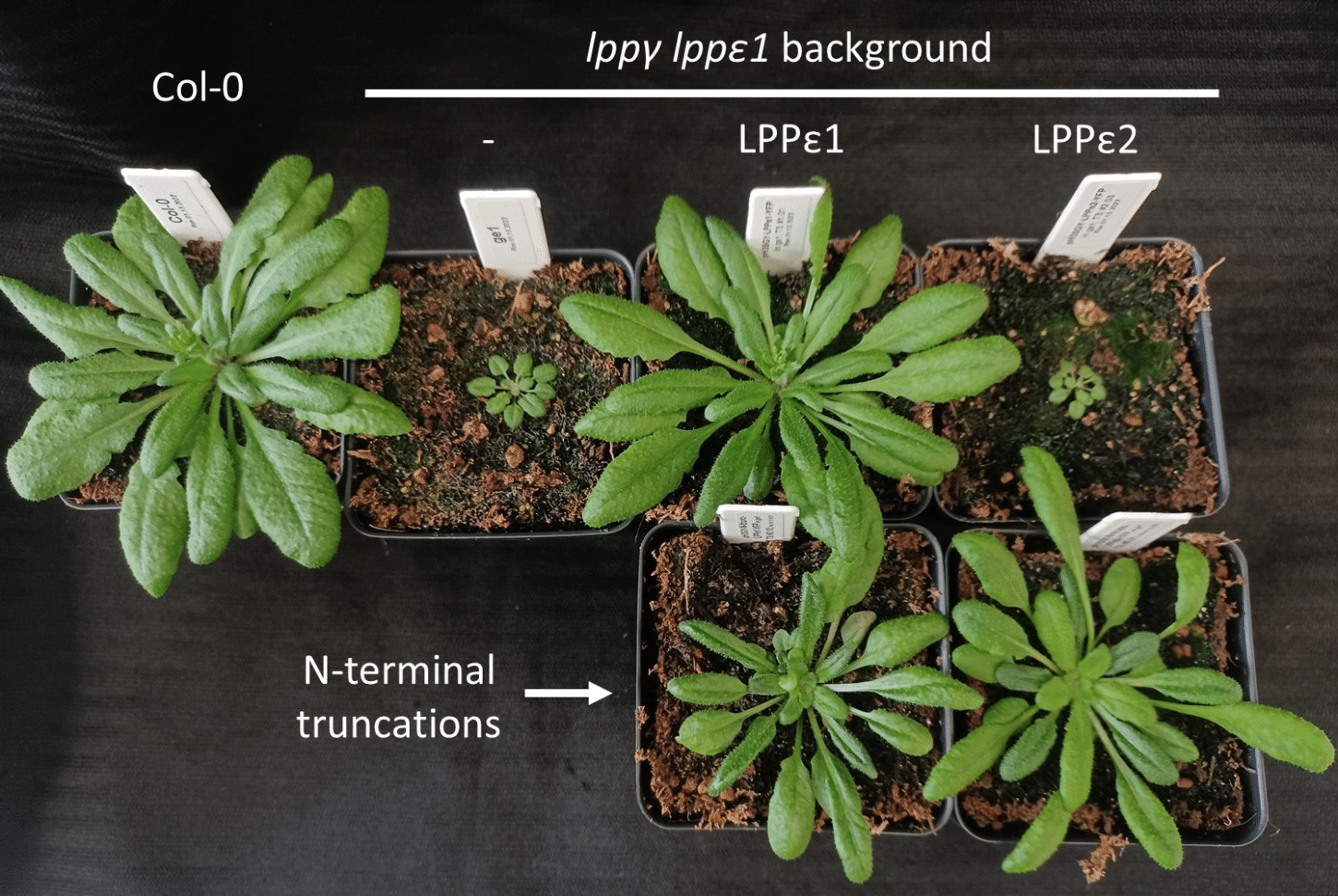
- LPPγ was previously thought to be essential to the growth of the plant. That is, any mutants lacking this enzyme would not grow. Yet, the Benning lab found they were able to grow these mutants without any problems.
- With their experiments, the researchers determined that two of the enzymes, LPPγ and LPPε1, were partially responsible for dephosphorylation of ER-derived phospholipids.
Photosynthesis is a complex process, involving many pathways and puzzle pieces working together to keep the organism alive.
In a recent study in Plant Physiology, Michigan State University researchers revealed a misconception that had been growing in plant science about one of the fundamental puzzle pieces of photosynthesis. In correcting the record, researchers can now think about using this knowledge to improve plant function and grow more efficient crops.
“Sometimes you just have to understand how the world works,” said Ron Cook, the first author of this study and a postdoctoral researcher in the MSU-Department of Energy Plant Research Laboratory, or PRL.
“It’s basic research,” said Cook, who works in the lab of Christoph Benning. “Once you understand how things work, then you could start thinking about how to change things and make them better.”
‘Nonviable’ proven viable
Courtesy photo
The paper focused on enzymes that help build the photosynthetic chloroplast membranes in plant cells.
One of those enzymes is known as LPPγ, or lipid phosphate phosphatase gamma.
LPPγ was previously thought to be essential to the growth of the plant. That is, any mutants lacking this enzyme would not grow. Yet, the Benning lab found they were able to grow these mutants without any problems.
“The paper essentially builds on a negative result correcting a previous misconception in the literature and points to a new direction to address an important biological question about chloroplast biochemistry,” said Benning, principal investigator of the study and director of the PRL.
Working with the surprisingly viable mutants led the investigators to look closer at two of LPPγ’s enzyme “cousins,” LPPε1 and LPPε2.
The role in the cell membrane
Photosynthesis happens in the chloroplast of plant cells, specifically in their photosynthetic membranes.
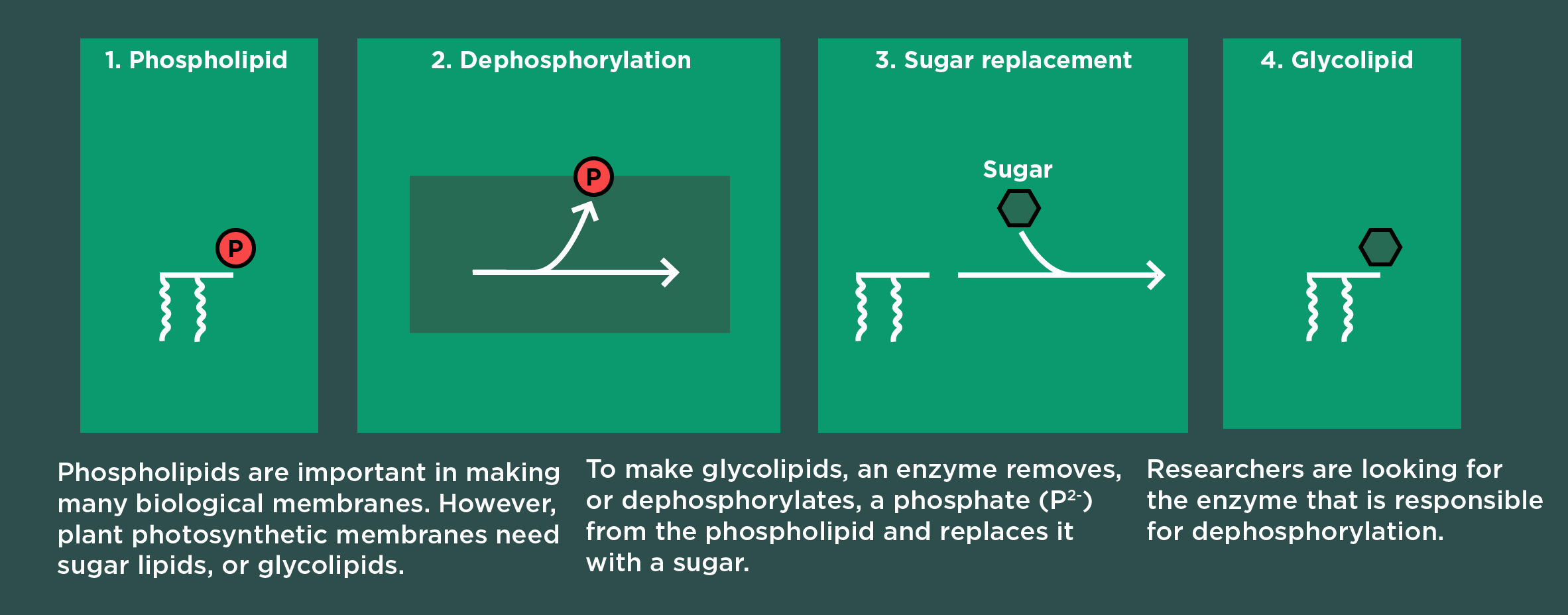
These differ from other membranes in biology. Most membranes have phospholipids. Plants’ photosynthetic membranes have sugar lipids instead. Researchers suspect plants made the switch because it decreases their dependence on phosphate nutrients in the soil.
And it is literally a switch: The membrane building blocks, or lipids, start out with phosphates. Plants use one enzyme to remove the phosphate and another enzyme to replace it with a sugar.
“It’s known which enzyme attaches that sugar after the phosphate has been removed, but less is known about which enzymes are responsible for removing the phosphate,” Cook said.
To see which enzymes play a part in this process, known as dephosphorylation, the researchers generated mutants of the plant Arabidopsis, a model organism used in research. Each of these mutant plants was missing two to three of the LPPγ, LPPε1 and LPPε2 enzymes thought to take part in this process.
Graphic by Abby Aldrich
Dephosphorylation can occur either within the chloroplast or outside of it, in what’s known as the endoplasmic reticulum, or ER. One of the mutants in the project struggled to convert those lipids made in the ER, Cook said.
With their experiments, the researchers determined that two of the enzymes, LPPγ and LPPε1, were partially responsible for dephosphorylation of ER-derived phospholipids.
“Two of these affect the import of lipids into the chloroplast and their combined function is essential for normal growth of the plant,” Benning said. “The function of the third homolog remains less certain.”
Looking for this unknown function is among the next steps for the researchers, continuing to unravel the mysteries of photosynthesis.
The research in this story is supported by the Office of Basic Energy Sciences of the United States Department of Energy (grant DE-FG02-91ER20021), Michigan State University AgBioResearch, and Michigan State University through a Training Program in Plant Biotechnology for Health and Sustainability funded by the National Institute of Health.



