Orange carotenoid proteins: structural understanding of evolution and function
Scientific Achievement
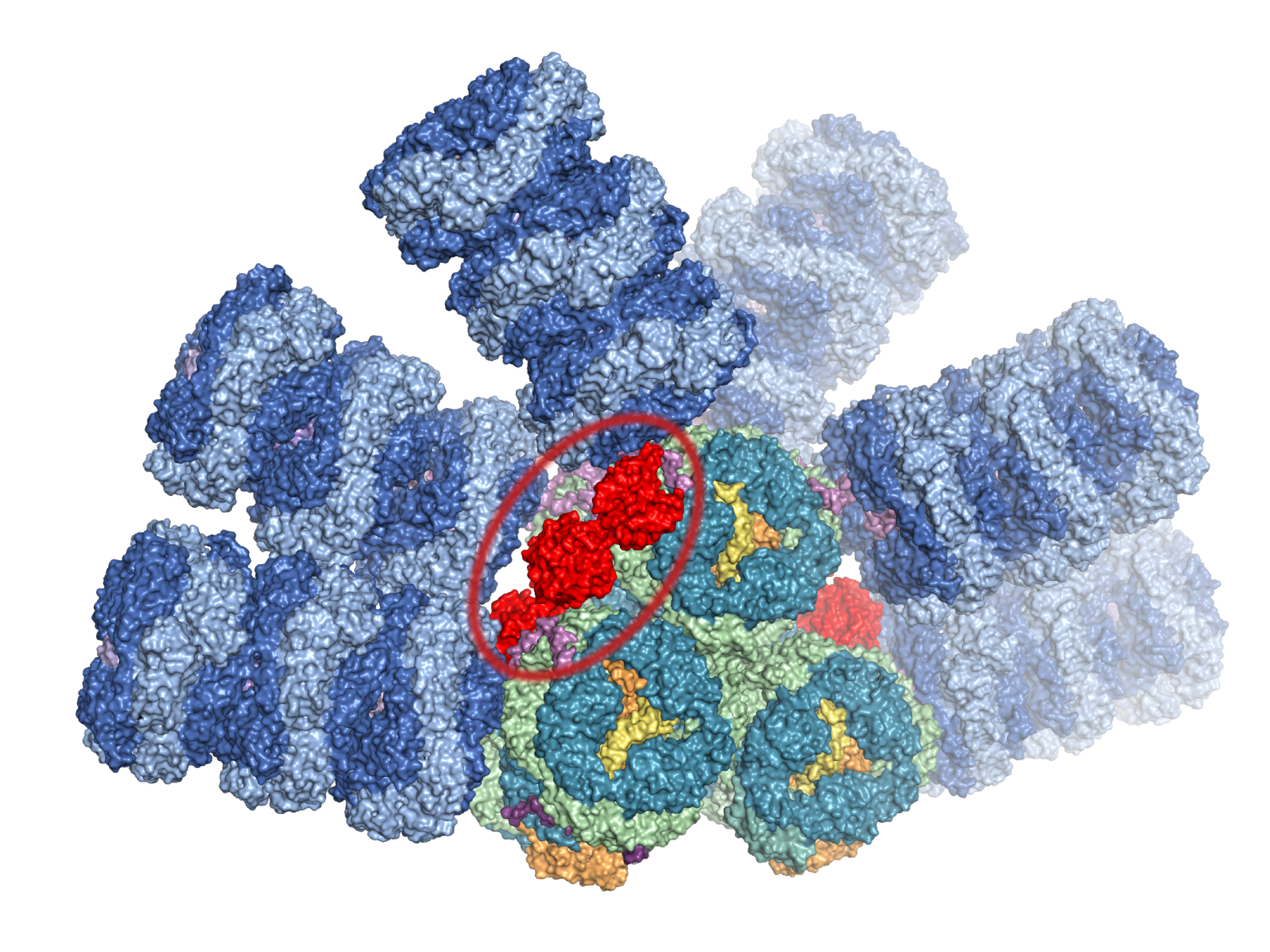
Cyanobacteria, photosynthetic microorganisms, contain a unique primitive water-soluble carotenoprotein, Orange Carotenoid Protein, or OCP. Carotenoids are organic orange pigments created in cyanobacteria.
This analysis and review suggests that the different families of the OCP use similar structural determinants for non-photochemical quenching, which is a process to dissipate excess captured energy under high light conditions that would damage the organism. The review also proposes a new model for how the Fluorescence Recovery Protein interacts with the OCP to begin to reversion of photoprotective quenching when the light levels return to ambient.
Significance and Impact
The quenching action of the active N-terminal domain of the OCP is conserved across Cyanobacteria with several instances of cross-species activity. Activity is also seen for some single domain homologs of the N-terminal of the OCP but the function of many of them, as well as the C-terminal domain homologs, remains enigmatic. The large structural change that the OCP undergoes when binding to the Phycobilisome antenna likely also has implications for the reversal of this process that is facilitated by the Fluorescence recovery protein.
Research Details
- Comparative analysis of homology models of distinct OCP family members
- Modeling of FRP-OCP interactions
- Review of recent insights into the evolution of the OCP
Related People: Cheryl A. Kerfeld and Markus Sutter
DOI: https://doi.org/10.1016/j.tibs.2024.04.010
Research in the Kerfeld laboratory was supported by the Office of Science of the US Department of Energy under awards DE-SC0020606 and DE-FG02-91ER20021, and with support from Michigan AgBio.



