Investigating the impacts of temperature on the thylakoid membrane: Insights from computer simulations
Article Highlights
- Environmental factors like temperature play a large role in how well a plant will grow and if it reaches its full potential
- Researchers from the Vermaas and Sharkey labs used computer simulations to investigate how isoprene levels and temperature affect the thylakoid membrane of plants at the nanoscale
- While isoprene concentrations did not have much effect, the membranes were sensitive to temperature
Environmental factors like temperature play a large role in how well a plant will grow and if it reaches its full potential. Using computer simulations, two lab groups at the MSU-DOE Plant Research Laboratory (PRL) teamed up to investigate how isoprene levels and temperature affect the thylakoid membrane of plants at the nanoscale.
This research is published in Plant, Cell & Environment.
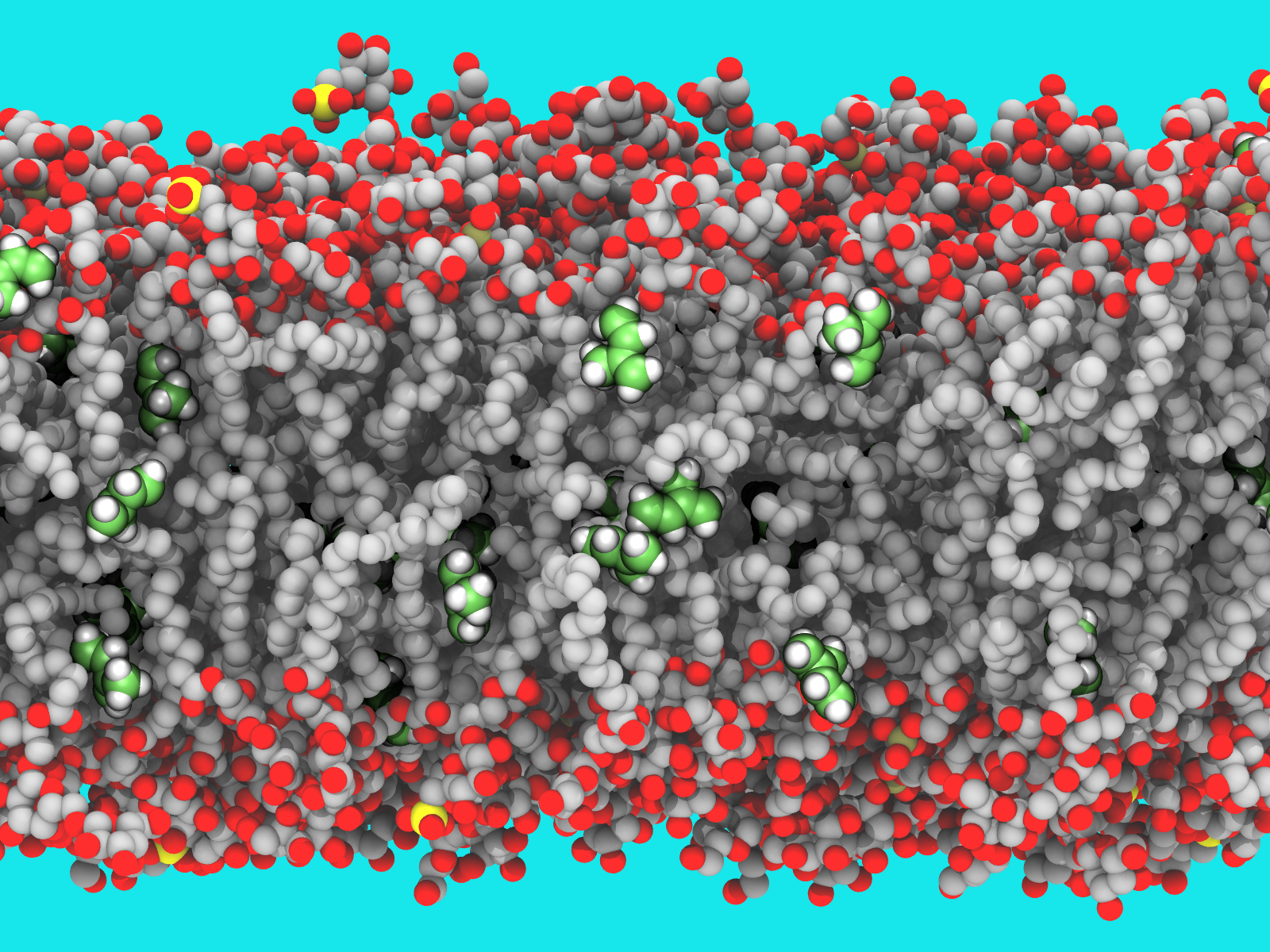
Image rendered with VMD by Josh Vermaas.
Isoprene is a gas emitted by many plants which has been shown to regulate photosynthesis, plant growth and resilience to biotic and abiotic stress, but it is not known how. One of the leading hypotheses is that isoprene would directly change the structure of the lipids found in plant thylakoid membranes, which contain the proteins active in photosynthesis.
Tom Sharkey, University Distinguished Professor at the PRL and in the Department of Biochemistry & Molecular Biology (BMB), approached the Vermaas lab with a question: How might isoprene affect thylakoid membranes in plants?
The lab, led by Assistant Professor Josh Vermaas, specializes in creating computer simulations to look at real-world issues. After creating an atomic model of the thylakoid membranes from existing data in scientific literature, the membranes were simulated at different isoprene concentrations. This allowed the researchers to make and track changes of the membrane’s properties under these conditions. The simulations quickly showed that the original hypothesis did not hold up.
“Although isoprene traveled inside the thylakoid membranes during the simulations, it didn't have much effect on the dynamical properties in the membrane,” said Martin Kulke, postdoctoral researcher in the Vermaas lab and first author of the study. “With the existing temperature data we collected during the simulations, we were able to rephrase our interest and looked at how temperature affects the thylakoid.”
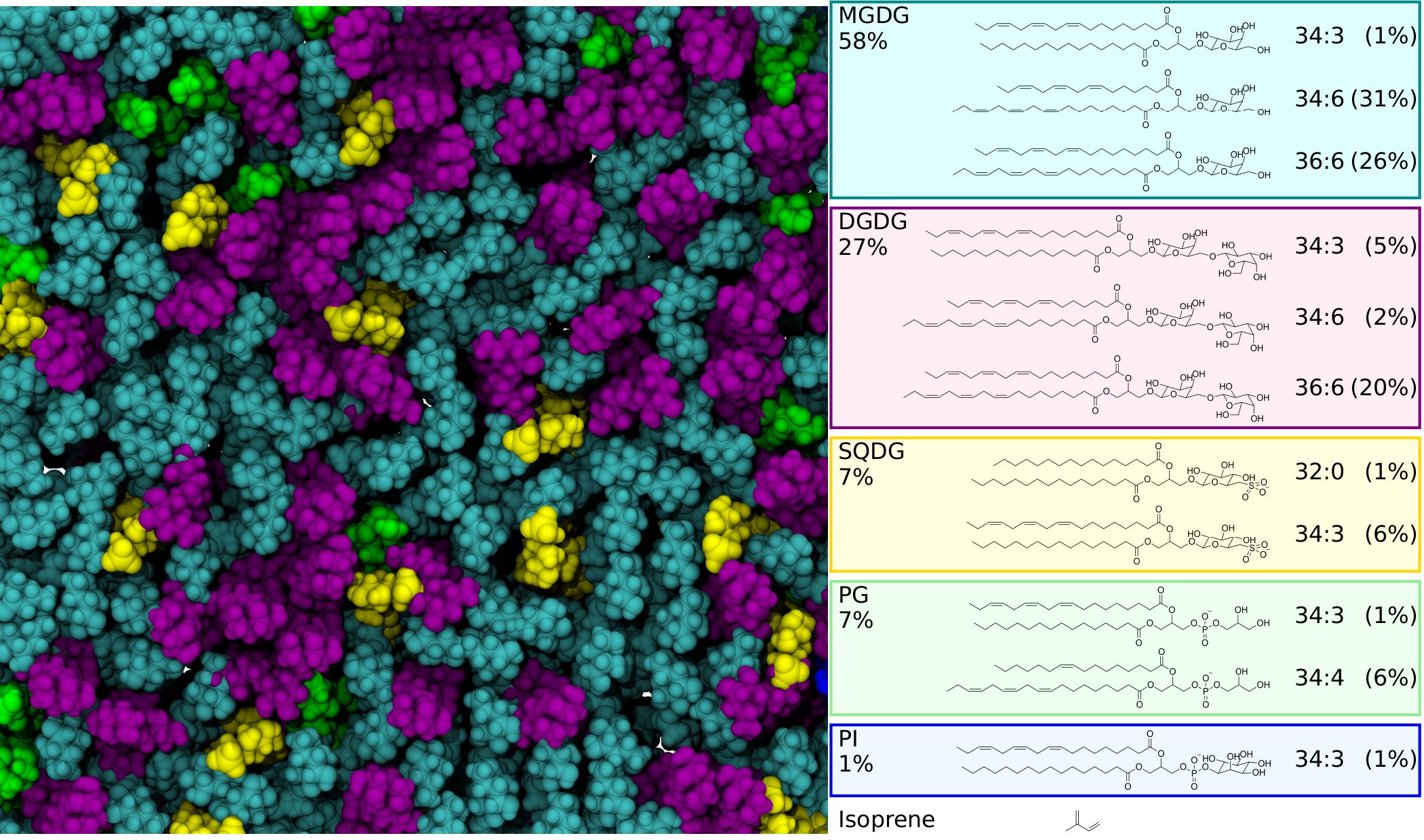
By Martin Kulke
Changing the temperature in the simulation showed that the light-harvesting complexes within the membranes might be sensitive to these changes. For example, as temperature increases, surface area, volume, flexibility and lipid diffusion increase, while membrane thickness decreases.
“The really big advantage of computer simulations is the level of control the scientist has,” said Vermaas, assistant professor in the PRL and BMB. “For us to test across multiple temperatures like we did in this study, we just needed to change a few critical lines to a configuration file and wait for our supercomputing resources to run the simulation under these new conditions.”
There are several experimental studies in scientific literature reporting how the composition and shape of thylakoids change with temperature, but only limited information on why these changes are occurring. Computer models can help to fill the gaps and develop hypotheses that are experimentally testable.
Additionally, in the field plants experience a multitude of environmental changes simultaneously. By using molecular simulations, the researchers can isolate what environmental changes are occurring to see how that condition specifically affects the plant.
Computer simulations are also less labor intensive, requiring no additional field or lab work. These advantages to using simulations help supplement the experimental data and can lead to new hypotheses to be tested, as shown through this collaboration.
“The work carried out by Martin Kulke and Josh Vermaas provided important insight into the biology of isoprene in plants and significantly increased our understanding in ways that required their rigorous, computational approach,” Sharkey said. “It is an excellent example of the PRL fostering collaborations that would be difficult in other settings.”
This research was supported by the Division of Chemical Sciences, Geosciences and Biosciences, Office of Basic Energy Sciences of the U.S. Department of Energy Grant DE-FG02-91ER20021, the U.S. National Science Foundation, Michigan AgBioResearch, the Institute for Cyber-Enabled Research and the Extreme Science and Engineering Discovery Environment project.
By Kara Headley; Banner image by Josh Vermaas



