Modifying a bacterial microcompartment shell proteins to bind metal ions for electron transfer with electrodes
The Kerfeld lab, in collaboration with the Naval Research Lab, has engineered a bacterial shell protein to incorporate copper for redox activity.
Scientific Achievement
Engineered a shell protein to incorporate copper for redox activity with an electrode surface.
Significance and Impact
Demonstrates the ability to expand the functionality of engineered bacterial microcompartments to non-native applications. Harnessing natural biological processes to synthesize new materials are key for developing future functional bioreactors and biomaterials.
Research Details
-
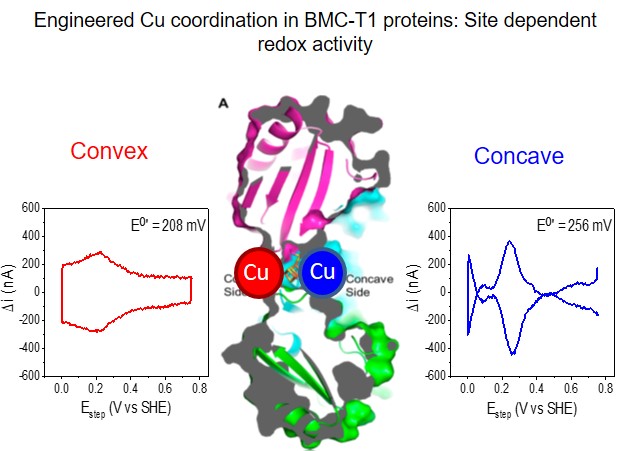
We designed, synthesized, and characterized a bacterial microcompartment shell protein for redox reactions by engineering either a Cu or [4Fe-4S] binding site.
-
Protein film voltammetry demonstrates tunable redox activity when the protein is attached to an electrode surface, which is preferable to solution state reactivity for many biomaterials applications.
Related people: Jefferson Plegaria, Matthew Yates, Sarah Glaven, Cheryl A. Kerfeld (CA)
This work was primarily funded by the US Department of Energy, Office of Basic Energy, with additional funding by the Naval Research Laboratory and the US Department of Defense.