New method tracks cyanobacteria photosynthetic productivity, in real time
MSU-DOE Plant Research Laboratory scientists have established a new method to quantify how much cyanobacteria assimilate carbon in the process of photosynthesis, in real time. The study is published in mBio.
Photosynthesis captures energy from sunlight in order to make carbohydrates, energy compounds that power life on Earth. The key ingredient in those compounds is carbon. That is why measuring how much carbon cyanobacteria assimilate is a good indicator of how productive they are.
The new method can assess carbon assimilation over a stretch of time. It also better factors in a wider range of environmental variables. Such variables include changing carbon dioxide (CO2) levels or varying light intensities.
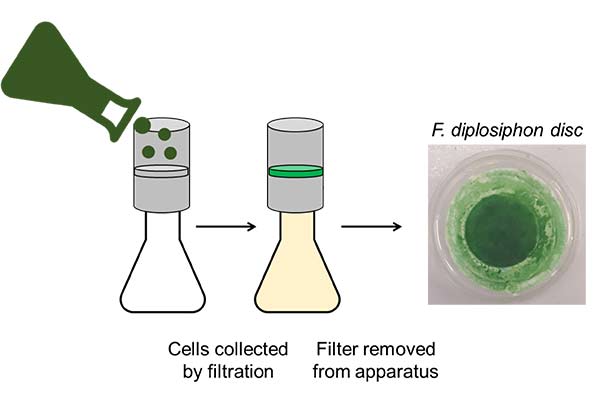
By the Montgomery lab, derived from mBio, CC BY 4.0
“Our method was enabled by Dr. David Hanson, whose team pioneered a way of putting aquatic, photosynthetic organisms on semi-wet filter discs. Having a solid disc allows us to measure the photosynthetic rates of algae and cyanobacteria in gas-exchange chambers, typically used for plant leaves,” says Brandon Rohnke, post-doc in the lab of Beronda Montgomery.
In these gas-exchange chambers, the cyanobacteria are fed set amounts of CO2. Within minutes, researchers can measure how much CO2 the organisms take up and assimilate.
Then, researchers develop, in real time, so-called carbon response curves. The curves help measure how much carbon the cyanobacteria can pick up from the air and have available to produce the energy compounds.
“Compared to current established methods, this new system makes it easier to monitor the carbon assimilation rate,” Brandon says. “We also can measure how cyanobacteria respond to shifts in environmental conditions, which is not as possible with the established methods.”
Improving on previous measuring systems
The Montgomery lab studies how small changes in cyanobacteria’s carbon processing system affects their productivity.
“We have a tough time examining minute changes in the carbon concentrating mechanism and determining how relevant they may be to an organism’s fitness and survival,” Brandon adds. “Even though we have a catalog of cyanobacteria strains that react differently to surrounding environmental conditions, we previously had limited methods to quantify how those changes affect a cell's actual ability to fix carbon.”
Established methods take hours to run, generally can only measure the endpoint outcome related to CO2 uptake and usage, and are challenging.
For example, one standard way is to grow cyanobacteria in a liquid culture. Then scientists measure how quickly the organisms release oxygen into the liquid. Since oxygen is a byproduct of photosynthesis, the measurement infers photosynthetic productivity. But, the result is indirect, a proxy.
Meanwhile, more direct methods can’t easily monitor how organisms adapt to changes in their environment. Everytime scientists want to measure the impact of a different concentration of carbon, using endpoint measures, they have to use a new batch of organisms. And liquid cultures take hours to reach a state of equilibrium where scientists can accurately measure CO2.
Contrastingly, the new method drastically reduces the technical and time challenges.
“The small amount of water on our solid disks allows for much more exposure to air. It takes two to five minutes for cyanobacteria to adjust to the levels of CO2 we have fed them. We perform the carbon response curves in a matter of minutes and can cover a range of CO2 levels.”
So, does it work?
The short answer: yes.
The Montgomery lab has tried the method with the cyanobacterium Fremyella diplosiphon, which has long-studied, distinct responses to red or green light availability.
The method reveals how light color, cell shape, and levels of photosynthetic pigments might impact how Fremyella assimilates CO2.
Specifically, green light encourages slightly higher assimilation levels, compared to red light.
“To test our method, we compared it with the established oxygen release method and managed to uncover novel aspects of carbon uptake versus its usage by cells. Another plus is that our measurements are very reproducible,” Brandon says.
“But we’d like to get to the point where we quantify specific photosynthetic variables.”
In other words, instead of just measuring how much carbon has disappeared from the atmosphere into the organisms, scientists want to discern where that carbon ends up in cyanobacterial cells and where there are any bottlenecks.
“It’ll take a while to get there, and more labs would need to adapt and contribute to developing the method,” Brandon says. “But as a relative measure to compare cyanobacteria strains with mutants, it’s already very effective and complements existing methods in the literature.”
This work was primarily funded by the US Department of Energy, Office of Basic Energy Sciences. Hero image: The Grand Prismatic Spring of Yellowstone National Park showing steam rising from hot water, which is surrounded by huge mats of brilliant orange algae and bacteria. By Brocken Inaglory (Own work) [CC BY-SA 3.0 or GFDL], via Wikimedia Commons.
By Igor Houwat, Brandon Rohnke



